Microspheres for High-Throughput Screening Assays

Contents
Introduction
Role of Microspheres
Current Microsphere-Based HIS Assays
Further Reading
Introduction (Back to Top)
An increased number of biological targets for drugs resulting from recent developments in genomic research, coupled with an increased efficiency in combinatorial library synthesis, have created a need for increasingly high-throughput screening (HTS) assays for drug discovery. A poll of HTS directors from several leading pharmaceutical companies regarding future trends for HTS assays' indicates:
- Targets screened will increase from current average of 18/year to 23/year by 2000.
- Currently, approximately 200,000 compounds are tested per screen; this is expected to increase to 300,000/screen in just two years.
- In 1998, absorbance/colorimetric techniques were the most often used detection mode in HTS.
- By 2003, the most prevalently used detection method will be fluorescence resonance energy transfer, including homogeneous time-resolved fluorescence.
- Cell-based assays will continue to replace some animal studies.
- A trend from heterogeneous to homogeneous assays.
- A trend toward outsourcing (now approximately 3/4 of HTS screening is done internally).
These assays have traditionally been carried out using radioactively labeled microspheres in the Scintillation Proximity Assay (Amersham), or by using 96-well plates as the solid phase. A trend away from radioactivity has created a need for a new type of detection. Similarly, a trend toward miniaturization has led to the need for a solid support that offers more sensitivity than the decreased surface area/well that 384- or 1536-well plates can offer. To solve these problems, companies are using various types of microspheres.
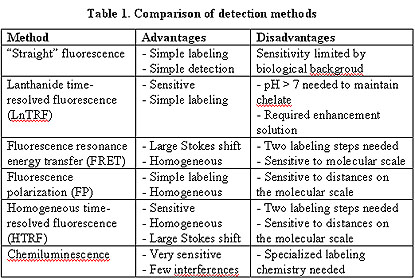
Fluorescence is being used as a replacement for radioactivity as a means of detection. Each of the fluorescent technologies listed in Table 1 can be enhanced by using microspheres to concentrate the dye, and thereby increase the fluorescent signal.
Role of Microspheres (Back to Top)
The role of microspheres in these screens is similar to their traditional role in immunoassays, namely as a solid phase to either enhance detection, separation, or both. The predominance of radioactive assays in high-throughput screening, along with the desire to find alternative means of detection, have led to research on substituting alternative fluorescent technologies. An example is a format using the same approach as the Scintillation Proximity Assay, but substituting fluorescence for radioactivity. This is commonly referred to as fluorescence resonance energy transfer (or, in the case of microspheres, fluorescence energy transfer latex), and can be diagrammed as in Figure 1.
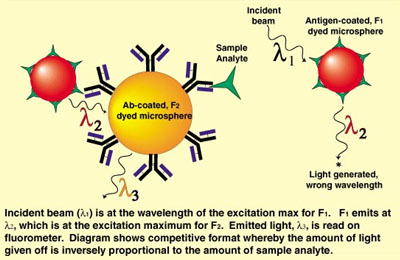
Figure 1
Several companies have researched assays using this microsphere-based technology. While most microspheres are internally dyed, the need for a close proximity for the energy transfer can necessitate using microspheres with the fluorescent dye covalently attached to the surface, instead. Bang's TechNote #19 describes fluorescent microspheres in more detail, and TechNote #13c lists protocols for covalently attaching different types of ligands to microspheres.
Table 2 shows various isotopic and non-isotopic means of detection, and their comparative sensitivities.
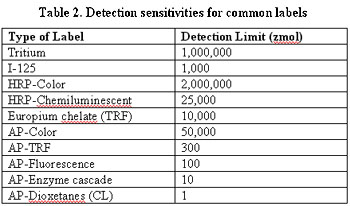
Table 2 illustrates that as the trend toward miniaturization continues in high-throughput screening assays, more sensitive means of detection will be needed. The added sensitivity provided by the increased surface area of microspheres vs. 96 (or more)-well plates will become more important to assay development.
Current Microsphere-Based HIS Assays (Back to Top)
There are already several companies using microspheres in their screening assays, both for biological and combinatorial chemical small molecule libraries. Dominic Spinella, director of exploratory research for Chugai Pharmaceuticals Inc., states that magnetic beads increase the sensitivity of his biological peptide screening assays by an order of magnitude over 96-well plates. Figure 2 illustrates how superparamagnetic microspheres were used for these assays.
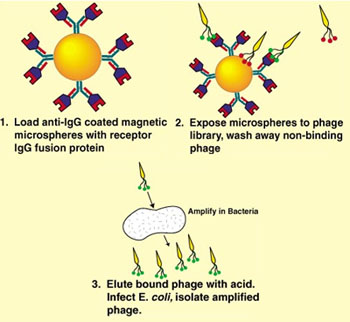
Figure 2
M13 phage infects male E. coli, and can be selectively mutated to express peptides of varying lengths at the surface. While these peptides might not be ideal drug candidates, they can give the basic structure, which, upon downstream modification, can become a drug. The fusion protein is a genetically engineered analog of the binding region of the target receptor. This is advantageous over traditional cell-based assays, in that isolating the target receptor can ensure that binding is due to the receptor of interest rather than other receptors at the cell surface.
Another example of microspheres being used for high-throughput screening assays involves using large, non-magnetic, streptavidin-coated microspheres that are trapped by filter-bottom plates. Using a protein kinase assay as an example, this approach can be diagrammed as shown in Figure 3.
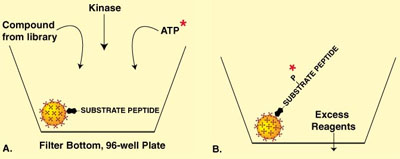
Figure 3
In Fig. 3A, the biotinylated kinase substrate peptide, which is commercially available, is attached to large, streptavidin-coated microspheres. Radioactively labeled ATP is mixed with the kinase, along with the potential agonist or antagonist from the compound library. Fig. 3B illustrates excess reagents being washed through the bottom of the filter-bottom plate. The amount of kinase activity is directly proportional to the scintillant count of the wells.
The advantage of streptavidin-coated microspheres in such an assay is both the increased sensitivity mentioned earlier and the ease of attachment of the biotinylated substrate peptide. This ease of attachment reduces costs both by saving time and eliminating the need for excess reagent usage. Protocols for the attachment of various types of ligands to streptavidin-coated microspheres can be found in Bang's TechNote #51.
A similar assay can be carried out on streptavidin-coated microspheres in standard 96-well plates, without using radioactivity. The example shown in Figure 4 is based on a chemiluminescent detection method, but could just as easily use a calorimetric detection method, by reacting alkaline phosphatase with a calorimetric substrate such as pNPP.
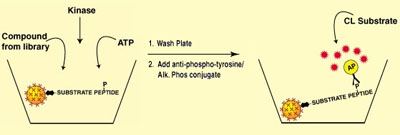
Figure 4
In this approach, ATP and kinase are added to the plate, which contains the biotinylated peptide substrate attached to streptavidin-coated microspheres, along with a potential agonist or antagonist from the compound library. The alkaline phosphatase conjugate yields quantitative calorimetric or chemiluminescent detection. The advantages to this approach are that the asasays are sensitive, non-isotopic, and detectable in concentrations analogous to in vivo kinase inhibitor concentrations. Commercial assays have been developed using a similar approach.
In addition to these specific applications, microspheres are now being used as the basis for entire high-throughput screening platform technologies, such as the electrochemiluminescent technology patented by IGEN International Inc., and now being used in their M-Series high-throughput screening instruments. This is a versatile technology using streptavidin-coated magnetic microspheres as the solid phase, and has already been implemented for use in drug discovery by several leading pharmaccutical companies.
- Fox, Sandra, Mary A. Yund, Shauna Farr-Jones, "Seeking Innovation in
High-Throughput Screening", R&D Magazine/Drug Discovery &
Development, 11/98, pp. 32-36.
- Bangs, LB, "Developing Inexpensive Tests and Assays Using Microspheres",
Workshop Notes, AACC Meeting New Orleans, July 19, 1994.
- Ikeda, K, et al., US Pat. #5,434,088.
- McConnell, J., "Biopanning Phage Display Libraries Using Magnetic Beads
vs. Polystyrene Plates", BioTechniques, 26, 2/99, pp. 208-214.
- Lehel, Csaba, et al, "A chemiluminescent Microtiter Plate Assay for Sensitive
Detection of Protein Kinase Activity", Analytical Biochemistry, 244, 1997,
pp. 340-346.
For more information: Joe Duffy, Technical Services, Bangs Laboratories Inc., 9025 Technology Drive, Fishers, IN 4603B-2BB6. Tel: 317-570-7020. Fax: 317-570-7034.
