Coulter, SmithKline submit Biologics License Application for Bexxar
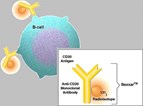
Radiopharmaceutical targets affected B-cells
Coulter Pharmaceutical Inc. (South San Francisco, CA) and SmithKline Beecham (Philadelphia) have re-submitted a Biologics License Application (BLA) to the U.S. Food and Drug Administration (FDA) for Bexxar (tositumomab, iodine I 131 tositumomab). The companies are seeking marketing approval of Bexxar for treating relapsed or refractory, low-grade or transformed low-grade B-cell non-Hodgkin's lymphoma (NHL).
Bexxar has been designated a Fast Track Product by the FDA because one of the targeted indications for the therapy is transformed, low-grade non-Hodgkin's lymphoma, a life-threatening disease for which no effective treatment exists. Along with Bexxar's Fast Track designation, the sponsoring companies will request a Priority Review.

Bexxar uses radioiodine to destroy affected B-cells in non-Hodgkins lymphoma. The isotope is conjugated to antibodies to B-cell surface antigens.
Bexxar is a radioimmunotherapy involving an antibody conjugated to iodine 131 (I-131) that attaches to a protein found only on the surface of B-cells, including non-Hodgkin's lymphoma B-cells. The properties of the I-131 radioisotope allow an appropriate patient-specific dose to be easily determined and administered. Bexxar is believed to work through multiple mechanisms of action resulting from immune system activity of the monoclonal antibody and the therapeutic effects of the cell-killing I-131 radioisotope. Since it targets tumor cells, those tissues receive a greater concentration of therapeutic radiation than normal cells.
Non-Hodgkin's lymphoma is a form of cancer that affects the blood and lymphatic tissues. NHL currently is the sixth leading cause of death among cancers in the United States and has the second fastest growing mortality rate. According to statistics from the National Cancer Institute, approximately 300,000 people are afflicted with NHL in the United States alone. It is estimated that approximately 140,000 people have low-grade or transformed low-grade disease, and 120,000 people have intermediate-grade disease.
For more information, contact Sylvia Wheeler of Coulter Pharmaceutical at 540-553-1887, or Jennifer Armstrong of SmithKline Beecham at 215-751-5664.
Edited by Angelo DePalma
Managing Editor, Drug Discovery Online
